Study participants
A total of 36 healthy adults were recruited for the study through notices posted at Inha University Hospital, Korea, from October 30, 2023, to November 7, 2023. The inclusion criteria were: (1) age between 20 and 60 years, and (2) provision of informed consent. Exclusion criteria were: those who had (1) chronic liver diseases (e.g., Hepatitis B or C virus carrier or liver cirrhosis), (2) previous history of coronary angioplasty and stenting due to coronary disease, (3) chronic kidney disease (estimated glomerular filtration rate < 60 ml/kg/1.73 m2), (4) any type of cancer, and (5) any psychiatric disorders. The sample size was determined based on Johanson and Brooks’ recommendation of 30 participants for a preliminary study, with a predicted dropout rate of 20%27. This study was approved by the Institutional Review Board of the Inha University Hospital (2023-05-016). This study was investigated in compliance with the Declaration of Helsinki. All the participants provided written informed consent.
Questionnaires
Information on participants’ demographics and plastic-related lifestyles was obtained using questionnaires that included the following items: sex, age, education (high school graduate, college graduate, or post-college), job (white-collar vs. blue-collar), marital status, smoking status (never, former, or current smoker), alcohol consumption, and physical inactivity. Physical inactivity was defined as moderate-to-vigorous physical activity for less than 150 min/week28. The plastic-related lifestyle questionnaires were screening questionnaires for environmental health clinics at Seoul National University Hospital, developed based on the references29,30. The questionnaire assessed the frequency of having ready-made meals (≥ 1/week or < 1/week), percentage of plastic containers among total containers in the refrigerator (≥ 50% or < 50%), percentage of discolored plastic container among total plastic containers (≥ 25% or < 25%), frequency of consuming vinyl-containing food (≥ 1/week or < 1/week), the frequency of consuming seafood (≥ 1/week, or < 1/week), and the frequency of indoor ventilation (≥ 1/day, < 1/day).
Blood sampling
Whole blood was obtained via venipuncture of the antecubital veins. Approximately 25.7 ml of blood was collected into a BD Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) 366,480 Sodium Heparin Glass tube, followed by Serum SST, EDTA, and Sodium citrate tubes. Samples in glass tubes for µ-FTIR analysis were stored at − 20 °C in a refrigerator for up to one week and then transferred to the Korea Institute of Analytical Science and Technology (KIAST) for µ-FTIR spectroscopy. KIAST conducted background contamination tests on the entire sampling system, including the needle and glass tubes. Blood samples were stored in a freezer at − 20 °C until analysis, for at least one week and at most eight weeks.
Extraction method
Preparation and analysis by µ-FTIR were performed by KIAST (https://www.kiast.co.kr/eng/main/main.php), an institute certified by the Korea Laboratory Accreditation Scheme (KOLAS). To remove organic matter from the blood, 30% H2O2 was added in 1 mL increments, carefully monitoring its rapid reaction with the blood. The total amount of added H2O2 was 30 mL. The mixture was shaken at 60 °C and 110 r/pm using a shaking incubator (Hanbaek Scientific Technology, HB-201SF) for a total of 2 weeks. Each blood sample (1 g) was placed in a prewashed flask using a glass Pasteur pipette. To remove organic matter from the blood, 30% H2O2 was added, and the mixture was shaken at 60 °C and 110 r/pm using a shaking incubator (Hanbaek Scientific Technology, HB-201SF). After adding 1 ml of 68% HNO3, the solution was filtered through a 5 μm silicon filter (10 mm × 10 mm square filters, pore diameter 5 μm, SmartMembrane, Germany). The flask was then washed with ultrapure water and filtered.
Analysis by µ-FTIR
One of the challenges in studying the health effects of microplastics on humans is exposure assessment31. Although markers indicating the exposure levels of MPs have been suggested, related studies are scarce. The samples were measured using µ-FTIR spectroscopy with a microscope (LUMOS II, Bruker Optics, USA) equipped with a 32 × 32-pixel focal plane array (FPA) detector. Infrared (IR) images were acquired in transmission mode with a spectral resolution of 8 cm− 1 and a single scan over the spectral range of 4000 to 750 cm− 1. The filter surface was obtained in Fig. S1a. Before IR imaging, sample photographs were taken to visualize surface morphology. Data analysis was performed using siMPle software, a freeware capable of rapidly detecting microplastics32. This software performs analyses using an algorithm that compares the IR spectrum of the sample with each reference spectrum in the database and assigns materials based on probability scores. The spectral library used in the analysis comprised spectra from polypropylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polystyrene (PS), polyvinylchloride (PVC), polyurethane (PU), polyamide (PA), poly(methyl methacrylate) (PMMA), and polycarbonate (PC). Plastic materials were classified into various plastic groups using the siMPle software. Samples with a major-to-minor axis ratio greater than 3 were classified as fibers, while the remaining samples were classified as fragments. The major diameters of the samples were used to classify them into five size classes: 5–10 μm, 10–20 μm, 20–50 μm, 50–100 μm, and > 100 μm.
Scanning electron microscopy
The morphology of particles collected on silicon filters was characterized using scanning electron microscopy (SEM) (JEOL JSM 7001 F, Japan). Before observation, the silicon filters were dried at 60 °C and sputter-coated with 7–10 nm of platinum to enhance image clarity. Images were acquired at an accelerating voltage of 15 kV, with magnifications ranging from 500 to 10,000 times, allowing for both overview and detailed examinations of the particles.
Analysis by µ-Raman
For the sample where MPs were not detected by µ-FTIR, a µ-Raman analysis was performed using an XploRA Plus confocal Raman microscope (Horiba, France) to verify blood samples that were not detected using µ-FTIR. A 532 nm laser with 10% decreased power and a 1024 × 256 pixel cooled charge-coupled device (CCD) detector were used. Grating with 1200 grooves/mm, a 100 μm confocal hole, and a 50 μm confocal slit width were set. A mosaic of microscopic dark field images of the filter surface was obtained, as illustrated in Fig. S1b. The mosaic image was processed using the Particlefinder™ module by Labspec 6 software, and all bright particles were selected against the dark background area. Raman spectrum was recorded by accumulating 1 × 2 s exposure time with a spectrum range between 1020 and 3400 cm1. A baseline correction of collected spectra was conducted using Labspec6 software. All spectra were screened for plastics using a classical least square algorithm (CLS). Each measured spectrum was calculated as the sum of all references and the theoretical composition. The results of the CLS Spectra were investigated manually to avoid missing or false positives by know-it-all software spectrum matching software with a library of Raman.
Quality control
To prevent microplastic contamination, all plastic tools were excluded, and glass and metal materials were used during the sampling and filtering processes. All glassware was washed with ultrapure water before use, and chemical reagents were used after being filtered through a 0.5 μm metal filter (KIAST, Korea). To prevent airborne microplastic contamination, sampling, pretreatment, and filtration steps were conducted on a laminar flow bench (HSCV-1300, SINAN Scientific Industry, Korea) inside a laminar flow cleaning booth. All samples were covered with aluminum foil when moved outside the laminar flow bench. Nitrile gloves and cotton coats were used to minimize sample contamination. Control samples were prepared for each sample set and pretreatment step to perform the same protocol and assess the potential impact of contamination.
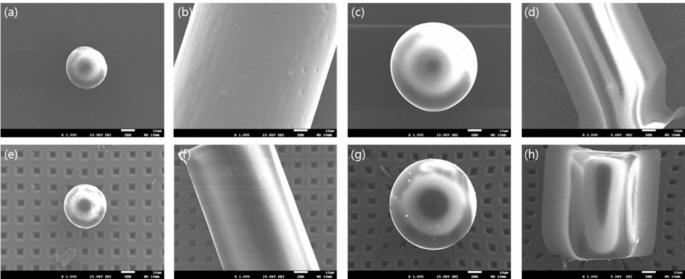
To evaluate the potential loss and damage that may occur during pretreatment, the recovery rate and surface morphology before and after pretreatment were compared using the same processes as those of the sample. Reference materials (RMs) of different sizes and types were used to distinguish between the intentionally added microplastic particles and those in the blood medium. Before spiking, RMs were dropped on the sliding glass to an appropriate number to manually count and confirm the shapes using a micro-Raman microscope and then injected into the blood. Fiber-shaped PP greater than 100 μm, average size of 27 μm PS sphere, and average size of 65 μm PS sphere were used for recovery testing. PET fiber greater than 100 μm was additionally added for damage testing. After the treatment of RMs spiked samples, the number was manually counted, comparing the FPA chemical image of µ-FTIR and the Raman microscopic image. Surface morphology was analyzed using an SEM (JEOL JSM 7001 F, Japan) to determine whether the chemical reagents damaged the microplastics after pretreatment. Surface morphology was analyzed using an SEM to determine whether the chemical reagents damaged the microplastics after pretreatment.
To evaluate potential contamination that may occur during the pretreatment of microplastics, procedural blank tests were conducted. A total of five blank samples were prepared when the sample batch was pretreated. Procedural blank samples that did not contain blood samples were treated using the same process as those used for blood samples. The results of the blank test were not subjected to any subtraction or correction but were intended to provide information regarding the environmental contamination of microplastics detected in the blood samples in this study.
Coagulation and inflammation markers
Blood collected using a Vacutainer Sodium Citrate tube was used to analyze prothrombin time (PT) and activated partial prothrombin time (aPTT). Fibrinogen was measured using an STA analyzer (Diagnostica Stago, Asnieres-Sur-Seine, France) within 4 h after blood collection. Antithrombin III activity was determined by an ELISA using assay kits (Diagnostica Stago). Blood collected by EDTA-anticoagulated Vacutainer Tube was used for platelet counts, which were measured using an automated analyzer (ADVIA 120; Siemens, Forchheim, Germany). Serum high-sensitive c-reactive protein (hsCRP) levels were measured by an immunonephelometric assay (Dade Behring Inc., Deerfield, IL, USA). StaRRsed Auto-Compact (Mechatronics Manufacturing BV, Zwaag, Netherlands) was used to measure the Erythrocyte sedimentation rate (ESR) with the Westergren sedimentation technique.
Statistical analysis
The differences in the detection of MPs and the number of total MPs by the characteristics of the participants and plastic-related lifestyle were tested using the Wilcoxon rank-sum test (or Kruskal-Wallis rank-sum test) and Chi-squared test, respectively. To compare the differences in the coagulation and inflammation markers by MP levels in blood, we divided the samples into two groups based on total MP particle counts: low (< 3/ml) and high (≥ 3/ml), according to the median value. Differences in hsCRP, PT, aPTT, antithrombin III, platelet count, ESR, and fibrinogen between the two groups were tested using the multivariate linear regression models with the adjustment for gender, age, education, job, marital status, smoking, alcohol, and physical inactivity. Additionally, we examined the beta coefficients (β) and P-values of the linear regression models between the number of total MP particles in the blood and each marker. All statistical analyses were performed using R version 4.2.3.